Plutonium
- Over one-third of the energy produced in most nuclear power plants comes from plutonium. It is created in the reactor as a by-product.
- Plutonium recovered from reprocessing normal reactor fuel is recycled as mixed-oxide fuel (MOX).
- Plutonium is the principal fuel in a fast neutron reactor, and in any reactor it is progressively bred from non-fissile U-238 that comprises over 99% of natural uranium.
- Plutonium has occurred naturally, but except for trace quantities it is not now found in the Earth's crust.
- There are several tonnes of plutonium in our biosphere, a legacy of atmospheric weapons testing in the 1950s and 1960s.
- Plutonium-238 is a vital power source for deep space missions.
In practical terms, there are two different kinds of plutonium to be considered: reactor-grade and weapons-grade. The first is recovered as a by-product of typical used fuel from a nuclear reactor, after the fuel has been irradiated ('burned') for about three years. The second is made specially for the military purpose, and is recovered from uranium fuel that has been irradiated for only 2-3 months in a plutonium production reactor. The two kinds differ in their isotopic composition but must both be regarded as a potential proliferation risk, and managed accordingly.
Plutonium, both that routinely made in power reactors and that from dismantled nuclear weapons, is a valuable energy source when integrated into the nuclear fuel cycle. In a conventional nuclear reactor, one kilogram of Pu-239 can produce sufficient heat to generate nearly 8 million kilowatt-hours of electricity.
Plutonium and nuclear power
Plutonium is formed in nuclear power reactors from uranium-238 by neutron capture. When operating, a typical 1000 MWe nuclear power reactor contains within its uranium fuel load several hundred kilograms of plutonium.
Reaction in standard UO2 fuel


Like all other heavy elements, plutonium has a number of isotopes, differing in the number of neutrons in the nucleus. All 15 plutonium isotopes are radioactive, because they are to some degree unstable and therefore decay, emitting particles and some gamma radiation as they do so.
All plutonium isotopes are fissionable with fast neutrons, though only two are fissile (with slow neutrons). For this reason all are significant in a fast neutron reactor (FNR), but only one – Pu-239 – has a major role in a conventional light water power reactor. Each fission yields a little over 200 MeV, or about 82 TJ/kg.
The main isotopes of plutonium are:
- Pu-238, (half-lifea 88 years, alpha decay to U-234, releasing 5.6 MeV)
- Pu-239, fissile (half-life 24,000 years, alpha decay to U-235)
- Pu-240, fertile (half-life 6,560 years, alpha decay to U-236)
- Pu-241, fissile (half-life 14.4 years, beta decay to Am-241)
- Pu-242, (half-life 374,000 years, alpha decay to U-238)
- (Periodic tables show an atomic mass of 244 for plutonium, suggesting Pu-244 as the most stable isotope with the longest half-life – 82 million years. It is the only one found in trace quantities in nature, apparently cosmogenic in origin from the formation of the Earth. It is not very relevant to this paper. It alpha decays to U-240.)
The most common plutonium isotope formed in a typical nuclear reactor is the fissile Pu-239, formed by neutron capture from U-238 (followed by beta decay), and which when fissioned yields much the same energy as the fission of U-235. Well over half of the plutonium created in the reactor core is 'burned' in situ and is responsible for about one-third of the total heat output of a light water reactor (LWR) and about 60% of the heat in a pressurized heavy water reactor (PHWR) such as CANDU. Of the rest in the LWR, about one-third through neutron capture becomes Pu-240 (and Pu-241). In a fast reactor this proportion is much less.
The approximately 1.15% of plutonium in the spent fuel removed from a commercial LWR power reactor (burn-up of 42 GWd/t) consists of about 53% Pu-239, 25% Pu-240, 15% Pu-241, 5% Pu-242 and 2% of Pu-238, which is the main source of heat and radioactivity.b British Magnox reactors used for production of military plutonium in their early years (to 1964) were run at about 0.4 GWd/t burn-up.
Examples of the types of variation in plutonium composition produced from different sources1
| Reactor type | Mean fuel burn-up (MW d/t) |
Percentage of Pu isotopes at discharge | Fissile content % |
||||
| Pu-238 | Pu-239 | Pu-240 | Pu-241 | Pu-242 | |||
| PWR | 33,000 | 1.3 | 56.6 | 23.2 | 13.9 | 4.7 | 70.5 |
| 43,000 | 2.0 | 52.5 | 24.1 | 14.7 | 6.2 | 67.2 | |
| 53,000 | 2.7 | 50.4 | 24.1 | 15.2 | 7.1 | 65.6 | |
| BWR | 27,500 | 2.6 | 59.8 | 23.7 | 10.6 | 3.3 | 70.4 |
| 30,400 | N/A | 56.8 | 23.8 | 14.3 | 5.1 | 71.1 | |
| CANDU | 7500 | N/A | 66.6 | 26.6 | 5.3 | 1.5 | 71.9 |
| AGR | 18,000 | 0.6 | 53.7 | 30.8 | 9.9 | 5.0 | 63.6 |
| Magnox | 3000 | 0.1 | 80 | 16.9 | 2.7 | 0.3 | 82.7 |
| 5000 | N/A | 68.5 | 25.0 | 5.3 | 1.2 | 73.8 | |
Plutonium-240 is the second most common isotope, formed by neutron capture by Pu-239 in about one-third of impacts. Its concentration in nuclear fuel builds up steadily, since it does not undergo fission to produce energy in the same way as Pu-239. (In a fast neutron reactor it is fissionablec, which means that such a reactor can utilize recycled plutonium more effectively than a LWR.) While of a different order of magnitude to the fission occurring within a nuclear reactor, Pu-240 has a relatively high rate of spontaneous fission with consequent neutron emissions. This makes reactor-grade plutonium entirely unsuitable for use in a bomb (see section on Plutonium and weapons below). Reactor-grade plutonium is defined as that with 19% or more of Pu-240. This is also called 'civil plutonium'.
Plutonium-238, Pu-240 and Pu-242 emit neutrons as a few of their nuclei spontaneously fission, albeit at a low rate. They and Pu-239 also decay, emitting alpha particles and heat.
A 1000 MWe light water reactor gives rise to about 25 tonnes of used fuel a year, containing up to 290 kilograms of plutonium. If the plutonium is extracted from used reactor fuel it can be used as a direct substitute for U-235 in the usual fuel, the Pu-239 being the main fissile part, but Pu-241 also contributing. In order to extract it for recycle, the used fuel is reprocessed and the recovered plutonium oxide is mixed with depleted uranium oxide to produce mixed oxide (MOX) fuel, with about 8% Pu-239 (this corresponds with uranium enriched to 5% U-235; see page on Mixed Oxide (MOX) Fuel).
Plutonium can also be used in fast neutron reactors, where a much higher proportion of Pu-239 fissions and in fact all the plutonium isotopes fission, and so function as a fuel. As with uranium, the energy potential of plutonium is more fully realized in a fast reactor. Four of the six 'Generation IV' reactor designs currently under development are fast neutron reactors and will thus utilize plutonium in some way (see page on Generation IV Nuclear Reactors). In these, plutonium production will take place in the core, where burn-up is high and the proportion of plutonium isotopes other than Pu-239 will remain high.
Developments under the Global Nuclear Energy Partnership (GNEP) make it very likely that the some military plutonium will be used in fast reactors in the USA (see page on International Framework for Nuclear Energy Cooperation).
In commercial power plants and research applications, plutonium generally exists as plutonium oxide (PuO2), a stable ceramic material with an extremely low solubility in water and with a high melting point (2,390 ºC). In pure form plutonium exists in six allotropic forms or crystal structure – more than any other element. As temperature changes, it switches forms – each has significantly different mechanical and electrical properties. One is nearly twice the density of lead (19.8 g/cm3). It melts at 640°C into a very corrosive liquid. The alpha phase is hard and brittle, like cast iron, and if finely divided it spontaneously ignites in air to form PuO2. Beta, gamma and delta phases are all less dense. Alloyed with gallium, plutonium becomes more workable.
In the USA, the early 1970s objectives of developing a ‘plutonium economy’ were derailed in the 1976 presidential campaign, and as a result fuel reprocessing to recover and recycle plutonium was banned until 2005 and fast reactor commercialization was aborted. Russia has maintained a positive policy of civil plutonium utilization.
Apart from its formation in today's nuclear reactors, plutonium was formed by the operation of naturally-occurring nuclear reactors in uranium deposits at Oklo in what is now west Africa, some two billion years ago.2
Plutonium and americium
Civil plutonium stored over several years becomes contaminated with the Pu-241 decay product americium-241 (see page on The Many Uses of Nuclear Technology), which interferes with normal fuel fabrication procedures. After long storage, Am-241 must be removed before the plutonium can be used in a MOX fuel fabrication plant because it emits intense gamma radiation (in the course of its alpha decay to Np-237).
Americium-241 from the UK plutonium stockpile is to be used by the European Space Agency. According to the National Nuclear laboratory (NNL), about 250 kg of old civil plutonium (originally with about 10-14% Pu-241) will yield 10 kg of Am-241, depending on its age – the half-life of Pu-241 is 14 years. The European Space Agency is paying NNL to produce Am-241 for 10-watt (e) radioisotope thermoelectric generators (RTGs) using very pure Am-241 recovered from old civil plutonium, as the isotope is much less expensive than Pu-238 (now scarce). Separation for plutonium is by dissolving plutonium dioxide in a silver-catalysed process, separating the plutonium from americium/silver, separating americium from silver and then recovering the silver. NNL hopes to make Am-241 a significant UK export.
Plutonium-238
Of some 2,900 types of radioisotopes known to humankind, only 22 are capable of powering a deep-space probe, according to a 2009 study by the US National Academy of Sciences. Of these, all but Pu-238 are problematical due to being too expensive, emitting too much radiation to work with, or lacking enough heat output (however, note European use of Am-241 in above section on Plutonium and americium).
The decay heat of Pu-238 (0.57 W/g) enables its use as an electricity source in the radioisotope thermoelectric generators (RTGs) of some cardiac pacemakers, space satellites, navigation beacons, etc. Its other physical properties enhance its usefulness: it forms a robust crystalline lattice, it has a high melting point – 2700°C – and it is insoluble in water.
Plutonium-238 has powered some 30 US space vehicles and enabled the Voyager 1 & 2 spacecraft to send back pictures of distant planets. These spacecraft have operated for over 35 years and are expected to send back signals powered by their RTGs through to 2025. The Cassini spacecraft carried three generators with 33 kg of plutonium oxide providing 870 watts power as it orbited around Saturn, having taken seven years to get there. The later and more efficient multi-mission RTG (MMRTG) uses eight 290-watt RTG units with a total of 4.8 kg plutonium-238 oxide producing 2 kW thermal, which can be used to generate some 110 watts of electric power, 2.7 kWh/day. It is being used in the NASA Mars Science Laboratory mission's rover Curiosity, which at 890 kg is about five times the mass of previous Mars rovers. Another MMRTG is set aside for the Mars 2020 rover. See also information page on Nuclear Reactors and Radioisotopes for Space.
Plutonium-238 is made by irradiating neptunium-237, recovered from research reactor fuel or special targets, in research reactors. Np-238 is formed and quickly decays to Pu-238. Both the reprocessing to obtain Np-237 and subsequent irradiation were carried out at Savannah River in USA. Pu-238 was then recovered by further reprocessing at the H Canyon plant there. The last of Savannah River’s neptunium inventory was transferred to Idaho National Laboratory (INL) in 2008. This was essentially Cold War-origin material.
Currently, supplies of high-purity Pu-238 are scarce. Since the early 1990s after production ceased at Savannah River in 1988, the USA was buying all its supply for spacecraft from Russia – some 16.5 kg, produced at Mayak – but Russia is no longer producing it and sales stopped about 2009.
After a gap of nearly 30 years, the USA restarted production of Pu-238 for NASA missions in December 2015. Oak Ridge National Laboratory (ORNL) is the lead laboratory for the project, in partnership with Los Alamos and Idaho National Laboratories. INL supplies the neptunium and does some of the irradiation. It uses the High Flux Isotope Reactor, irradiating neptunium-237 targets for 72 days. The plutonium is then chemically separated and purified to produce an oxide powder. ORNL expects production to ramp up to 1.5 kg/yr by the mid-2020s.
In February 2017 Ontario Power Generation (OPG) and its venture arm, Canadian Nuclear Partners, announced plans to produce Pu-238 for space exploration at the Darlington nuclear power plant and signed a contract for this with NASA. OPG would use a similar process to that at its Pickering units to produce cobalt-60. The process was developed by Technical Solutions Management (TSM), which would also manage the project. In this, Np-237 targets would be made by DOE’s Pacific Northwest National Laboratory (PNNL) and shipped to Chalk River Laboratories in Ontario to be assembled into reactor bundles. These would be irradiated at Darlington then returned to Chalk River for processing. Production target is reportedly 5 kg Pu-238 per year by about 2022, but the project is yet to receive regulatory approval.
Early heart pacemakers used Pu-238 as the power source, and after 30 years some were still running well.
Plutonium and weapons
It takes about 10 kilograms of nearly pure Pu-239 to make a bomb (though the Nagasaki bomb in 1945 used less). Producing this requires 30 megawatt-years of reactor operation, with frequent fuel changes and reprocessing of the 'hot' fuel. Hence 'weapons-grade' plutonium is made in special production reactors by burning natural uranium fuel to the extent of only about 100 MWd/t (effectively three months), instead of the 45,000 MWd/t typical of LWR power reactors. Allowing the fuel to stay longer in the reactor increases the concentration of the higher isotopes of plutonium, in particular the Pu-240 isotope, as can be seen in the Table above. For weapons use, Pu-240 is considered a serious contaminant, due to higher neutron emission and higher heat production. It is not feasible to separate Pu-240 from Pu-239.
The operational requirements of power reactors and plutonium production reactors are quite different, and so therefore is their design. No weapons material has ever been produced from PWR, BWR, or PHWR power reactors (96% of the worldwide fleet by capacity). An explosive device could be made from plutonium extracted from low burn-up reactor fuel (i.e. if the fuel had only been used for a short time), but any significant proportions of Pu-240 in it would make it hazardous to the bomb makers, as well as probably unreliable and unpredictable. Typical 'reactor-grade' plutonium recovered from reprocessing used power reactor fuel has about one-third non-fissile isotopes (mainly Pu-240)d.
In the UK, the Magnox reactors were designed for the dual use of generating commercial electricity as well as being able to produce plutonium for the country's defence programme. A report released by the UK's Ministry of Defence (MoD) says that both the Calder Hall and the Chapelcross power stations, which started up in 1956 and 1958 respectively, were operated on this basis3. The government confirmed in April 1995 that production of plutonium for defence purposes had ceased in the 1960s at these two stations, which are both now permanently shutdown. The other UK Magnox reactors were civil stations subject to full international safeguards.
International safeguards arrangements applied to traded uranium extend to the plutonium arising from it, ensuring constant audits even of reactor-grade material. This addresses uncertainty as to the weapons proliferation potential of reactor-grade plutonium. There is no uncertainty that such material can be made to explode, though there is no known occasion when it has been exploded (a 1962 US test using UK plutonium from its Magnox reactors had a relatively high level of Pu-240 but evidently less than ‘reactor grade’ as subsequently defined).
Plutonium in the reactor core

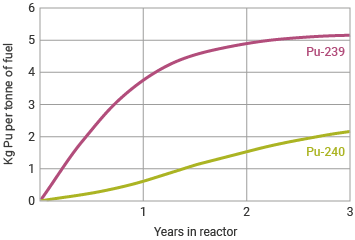
The International Atomic Energy Agency (IAEA) is conservative on this matter so that, for the purpose of applying IAEA safeguards measures, all plutonium (other than plutonium comprising 80% or more of the isotope Pu-238) is defined by the IAEA as a 'direct-use' material, that is, "nuclear material that can be used for the manufacture of nuclear explosives components without transmutation or further enrichment". The 'direct use' definition applies also to plutonium which has been incorporated into commercial MOX fuel, which as such certainly could not be made to explode.
The following Table contrasts the plutonium or plutonium mixture separated out from three different fuel cycles: short cycle/low burn-up uranium fuel, normal high burn-up uranium fuel, and high burn-up fast reactor fuel. As can be discerned from the attributes of each, it is the first which produces weapons-usable material.
| Type | Composition | Thermal power w/kg | Spontaneous neutrons /s/g | Origin | Use |
| Weapons-grade | Pu-239 with less than 8% Pu-240 | 2-3 | 60 | From military 'production' reactors with metal fuel operated for production of low burn-up Pu. Purex separation. | Nuclear weapons (can be recycled as fuel in fast neutron reactor or as ingredient of MOX) |
| Reactor-grade from high-burnup fuel | 55-70% Pu-239; more than 19% Pu-240 (typically about 30-35% non-fissile Pu) | 5-10 | 200 | Comprises about 1% of used fuel from normal operation of civil nuclear reactors with oxide fuel used for electricity generation | As ingredient (c. 5-8%) of MOX fuel for normal reactor |
| IFR-grade actinide | Pu + minor actinides + U, 50% Pu fissile | 80-100 | 300,000 | From fast reactor used metal fuel by pyroprocessing | recycle |
The US DOE Plutonium Disposition Working Group in April 2014 defined weapons plutonium as comprising less than 10% Pu-240 relative to Pu-239. It quoted Rosatom reporting that used MOX fuel from the BN-800 fast reactor was more than 17% Pu-240.
Resources of plutonium
Total world generation of reactor-grade plutonium in spent fuel is some 70 tonnes per year. About one-third of the separated Pu has been used in mixed oxide (MOX) fuel. Currently over 1000 tUeq of Pu is used in MOX each year (see page on Mixed Oxide (MOX) Fuel).
Three US reactors are able to run fully on MOX, as can Canadian heavy water (CANDU) reactors. All Western and the later Russian light water reactors can use 30% MOX in their fuel. About 40 European reactors are licensed to use MOX fuel, and several in France are using it as 30% of their fuel. Areva's EPR design is capable of running a full core load of MOX.
The UK's plutonium stockpile is 139 tonnes of separated civil plutonium from historic and current operations and foreign swaps.
At the end of 2019 France had about 75 tonnes of separated civil plutonium stored domestically. Some 10.5 tonnes of plutonium and 1000 tonnes of reprocessed uranium (RepU) are recovered each year from the 1050 tonnes treated each year. The plutonium is immediately shipped to the 195 t/yr Melox plant near Marcoule for prompt fabrication into about 100 tonnes of MOX fuel.
Japan at the end of 2019 had about 9 tonnes of separated civil plutonium stored domestically, plus 36.69 t held abroad in the UK and France.
Russia at the end of 2019 had about 63 tonnes of separated civil plutonium stored domestically.
The USA had no reactor-grade plutonium separated, but had at the end of 2019 about 45 tonnes of weapons-grade material destined for MOX.
China at the end of 2016 had about 41 tonnes of separated civil plutonium. India’s plutonium stocks are unknown. Worldwide stocks of civil plutonium are estimated as around 260 tonnes.
In June 2000, the USA and Russia agreed to dispose of 34 tonnes each of weapons-grade plutonium by 2014. Construction on the Mixed Oxide Fuel Fabrication Facility at the Savannah River Site near Aiken, South Carolina commenced in August 2007. The plant was designed to dispose of 34 tonnes of weapons-grade plutonium by converting it into MOX fuel, but the US DOE terminated the contract in October 2019 with the facility 70% complete. Earlier in October 2016 Russia suspended the agreement with the USA.
Generation IV reactor designs are under development through an international project. Four of the six designs are fast neutron reactors and will thus utilize plutonium in some way. In these, plutonium production will take place in the core, where burn-up is high and the proportion of plutonium isotopes other than Pu-239 will remain high.
See also page on Military Warheads as a Source of Nuclear Fuel.
Toxicity and health effects
Despite being toxic both chemically and because of its ionising radiation, plutonium is far from being "the most toxic substance on Earth" or so hazardous that "a speck can kill". On both counts there are substances in daily use that, per unit of mass, have equal or greater chemical toxicity (arsenic, cyanide, caffeine) and radiotoxicity (smoke detectors).
There are three principal routes by which plutonium can get into human beings who might be exposed to it:
- Ingestion.
- Contamination of open wounds.
- Inhalation.
Ingestion is not a significant hazard, because plutonium passing through the gastro-intestinal tract is poorly absorbed and is expelled from the body before it can do harm.
Contamination of wounds has rarely occurred although thousands of people have worked with plutonium. Their health has been protected by the use of remote handling, protective clothing and extensive health monitoring procedures.
The main threat to humans comes from inhalation. While it is very difficult to create airborne dispersion of a heavy metal like plutonium, certain forms, including the insoluble plutonium oxide, at a particle size less than 10 microns (0.01 mm), are a hazard. If inhaled, much of the material is immediately exhaled or is expelled by mucous flow from the bronchial system into the gastro-intestinal tract, as with any particulate matter. Some however will be trapped and readily transferred, first to the blood or lymph system and later to other parts of the body, notably the liver and bones. It is here that the deposited plutonium's alpha radiation may eventually cause cancer.
However, the hazard from Pu-239 is similar to that from any other alpha-emitting radionuclides which might be inhaled. It is less hazardous than those which are short-lived and hence more radioactive, such as radon daughters, the decay products of radon gas, which (albeit in low concentrations) are naturally common and widespread in the environment.
In the 1940s some 26 workers at US nuclear weapons facilities became contaminated with plutonium. Intensive health checks of these people have revealed no serious consequence and no fatalities that could be attributed to the exposure. In the 1990s plutonium was injected into and inhaled by some volunteers, without adverse effects. In the 1950s Queen Elizabeth II was visiting Harwell and was handed a lump of plutonium (presumably Pu-239) in a plastic bag and invited to feel how warm it was.
Plutonium is one among many toxic materials that have to be handled with great care to minimize the associated but well understood risks.
Notes & references
Notes
a. Half-life is the time it takes for a radionuclide to lose half of its own radioactivity. The fissile isotopes can be used as fuel in a nuclear reactor, others are capable of absorbing neutrons and becoming fissile (i.e. they are 'fertile'). Alpha decays are generally accompanied by gamma radiation. [Back]
b. Comparable isotopic ratios are found in the spent fuel of CANDU heavy water reactors at much lower burnups (8 GWd/t), due to their use of natural uranium fuel and high thermal neutron spectrum. From gas graphite Magnox reactors the plutonium has more Pu-239 – about 65%, plus 25% Pu-240, 5% Pu-241, 1% Pu-242 and negligible Pu-238. [Back]
c. The term 'fissionable' applies to isotopes that can be made to undergo fission. If a fissionable isotope only requires neutrons with low kinetic energy to undergo fission, then it is said to 'fissile'. Thus, all fissile isotopes are fissionable. Pu-240 is fissionable, as it undergoes fission in a fast neutron reactor – but it is not a fissile isotope. [Back]
d. In 1962 a nuclear device using low-burnup plutonium from a UK Magnox reactor was detonated in the USA. The isotopic composition of this plutonium has not been officially disclosed, but it was evidently about 85% Pu-239 – what would since 1971 have been called 'fuel-grade' plutonium. The plutonium used in the bomb test was almost certainly derived from the Calder Hall/Chapelcross reactors then operating as military plutonium production reactors (see Reference 3 below). As part of the UK's 1998 Strategic Defence Review, a UK Ministry of Defence document stated: "The US Government has given assurances that UK plutonium transferred to the US since 1964 was not used in the US nuclear weapons programme. It is theoretically possible, but very unlikely, that some UK civil plutonium may have been transferred to the US and used in the US nuclear weapons programme before 1964." [Back]
References
1. Data taken from NDA Plutonium Options, Nuclear Decommissioning Authority (2008). [Back]
2. Information on the Oklo natural reactors is on the Swedish Nuclear Fuel and Waste Management Company (Svensk Kärnbränslehantering, SKB) website (www.skb.se). See also I. Gurban and M. Laaksoharju, Uranium transport around the reactor zone at Okelobondo (Oklo), Data evaluation with M3 and HYTEC, SKB Technical Report TR-99-36 (December 1999). [Back]
3. Plutonium and Aldermaston - An Historical Account, UK Ministry of Defence (2000). [Back]
General sources
HV Henderickz, Plutonium: blessing or curse?, Copper Beech 1998 (ISBN: 9782930221083)
Management of Separated Plutonium - The Technical Options, NEA/OECD Paris 1997 (ISBN: 9789264154100)
Management of separated plutonium, The Royal Society, February 1998 (ISBN: 9780854035144)
Plutonium Fuel: An Assessment, OECD/NEA Paris 1989 (ISBN: 9789264132658)
Plutonium Management in the Medium Term, A Review by the OECD/NEA Working Party on the Physics of Plutonium Fuels and Innovative Fuel Cycles (WPPR), OECD/NEA Paris 2003 (ISBN: 9264021515)
Managing the Plutonium Surplus: Applications and Technical Options, NATO 1994 (ISBN 9780792331247)
The Toxicity of Plutonium, Medical Research Council, HMSO London 1975 (ISBN: 0114500304)
Plutonium articles in Revue Generale Nucleaire, June 1995
D Fishlock, Drama of Plutonium, Nuclear Engineering International (2005)
J Carlson, Introduction to the Concept of Proliferation Resistance, International Commission on Nuclear Non-proliferation and Disarmament (ICNND, January 2009). See also the website of the ICNND (www.icnnd.org) for more information on nuclear non-proliferation
Radioisotope Power Systems: An Imperative for Maintaining U.S. Leadership in Space Exploration, US National Academy of Sciences (2009)
Mitch Ambrose, Oak Ridge scientists produce first plutonium-238 in 28 years, Physics Today (4 February 2016)
Ethan Siegal, NASA Doesn’t have enough nuclear fuel for its deep space missions, Forbes (13 December 2018)
Related information
Nuclear Fuel Cycle OverviewMixed Oxide Fuel MOX
Processing of Used Nuclear Fuel
Japanese Waste and MOX Shipments From Europe
Military Warheads as a Source of Nuclear Fuel
International Framework for Nuclear Energy Cooperation